A few years ago, Dr. Ian McGilvray found that he could safely and more easily transplant livers if he first removed a small piece of the organ that obscures a major blood vessel.
What he could not have anticipated was how these leftover surgical scraps would unleash a flurry of discovery that is shedding new light on the elusive organ and strengthening Toronto as a global centre for liver research.
“The amazing thing about the human liver is that it’s 2019 and we don’t really understand it”, says McGilvray, a surgeon and research director of the transplant program at University Health Network (UHN), and an associate professor in the University of Toronto’s (U of T) Department of Surgery. “The problem with the human liver fundamentally is a lack of access to it.”
Previously, researchers had to make do by studying animals or samples taken from patient biopsies, but neither of these represented a healthy human liver. By removing the caudate lobe, which is the size of “a jalapeño pepper” and not crucial for liver function, McGilvray not only made transplants easier to do, but also created a regular supply of normal liver tissue for researchers in Toronto to study.
Through U of T’s Medicine by Design initiative, which is accelerating the development of regenerative medicine therapies through collaborative research, McGilvray made connections with experts in single-cell genomics — a revolutionary new technology that maps which genes are switched on or off in individual cells in a tissue — just as they were getting the method off the ground in Toronto. Together, they have identified all cell types in the liver to create the first molecular map of any organ in the human body, a discovery they described recently in a paper in Nature Communications.
By revealing the molecular make up of all cell types in the liver, this map is a long-awaited guide for two Medicine by Design team projects that are looking to recreate liver cells in the lab for research and therapy.
Read more about how the world’s first human liver map came together
One team is led by stem cell pioneer Gordon Keller, director of the McEwen Stem Cell Institute at UHN and a professor in U of T’s Department of Medical Biophysics, who is collaborating with McGilvray on a Medicine by Design team project and is already using the liver map data to grow different types of liver cells in the lab.
“Our primary goal is to grow the different types of liver cells from stem cells for the purpose of developing new therapies to treat liver disease,” says Keller, who leads the interdisciplinary Medicine by Design research team.
“We can use these new data as a molecular guide. We can look at the make-up of the normal liver and ask how close are our cells to the normal liver cells,” says Keller.
Besides McGilvray, Keller’s Medicine by Design liver team also includes liver disease specialists and engineers whose combined expertise will make it easier to clear hurdles toward their ambitious goal.
Another collaborative Medicine by Design team is led by UHN’s Jason Fish, a senior scientist at the Toronto General Hospital Research Institute (TGHRI) and an associate professor at U of T’s Department of Laboratory Medicine & Pathobiology. It, too, is relying on insights from the liver map to grow tissue that could be used to boost survival of donor livers.
The two teams are among the 19 collaborative teams Medicine by Design is funding from across U of T and its affiliated hospitals to accelerate the translation of regenerative medicine discoveries into new therapies for patients.
The body’s “seat of the soul”
Known since ancient times as “the seat of the soul”, the liver plays many vital roles: it cleans blood, helps digest food and makes essential proteins for the body. Weighing about two kilograms, the liver also has an uncanny ability to regenerate; if cut to a quarter of its size, the whole organ will grow back in as little as six weeks.
Read more about “the blob that runs the body” in The New York Times
Despite this rejuvenating capacity, Western diets awash with processed food have been disastrous for the liver, with cases of liver cancer and fatty liver disease skyrocketing in Canada and around the world. If the liver becomes damaged beyond repair, a transplant is the only hope. And with donated organs in scarce supply, many patients do not live long enough to receive one. In 2017, 530 liver transplants were carried out in Canada, but 71 people died while waiting for a liver to become available and. By the end of the year, 418 people were still on the wait list, according to a report from the Canadian Institute for Health Information.
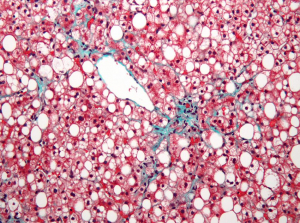
Blobs of fat (white circles) fill up and distort hepatocytes, stained in red, which up bulk of the liver. Cases of fatty liver disease are on the rise in Canada. (Nephron via Wikimedia Commons)
Even when a liver becomes available, it has to be transplanted as soon as it is removed from the body for the transplant to succeed.
With stem cells, researchers hope to boost transplant success rates and even develop new treatments that could avoid the need for donor organs altogether. For example, it may be possible to grow replacement tissue from stem cells to graft on to a diseased liver. Another approach could be to enhance the liver’s natural ability to heal. Perhaps one day researchers could even take out the sick liver, fix it outside the body and put it back into the patient as good as new.
If anyone knows how to create tissue in the lab, it’s Keller. Over the years, his team has developed cell culture methods that re-enact the stem cells’ unique microenvironment in the developing embryo to coax them to become diverse specialized cells. They have already created transplantable heart cells that can repair heart damage in experimental animals, paving the way for developing potential new therapies to treat heart disease. As part of another Medicine by Design team, Keller is producing blood from stem cells to make treatments for blood disorders more widely available.
As the leader of one of the two Medicine by Design liver teams, Keller seeks to grow the major liver cell types for a variety of liver problems. Among these are hepatocytes, which make the bulk of the organ and drive its regeneration. Another are cholangiocytes, cells that line the inside of the bile ducts and transport bile, a dark-coloured fluid that is important for digestion.
Failure to move bile properly can lead to illnesses that can be cured only by getting a new liver. If functional bile ducts could be grown in the lab, it’s possible they could be transplanted instead of whole organs.
Keller’s team has already succeeded in growing cholangiocytes from stem cells. Now they need the tubes for the bile to flow through.
This is where Axel Guenther’s engineering team comes in. Guenther, an associate professor in the Department of Mechanical and Industrial Engineering, is an expert in bioprinting, which combines cells, growth factors and biomaterials to mimic a tissue’s three-dimensional geometry in the body.
Guenther has developed a method for making tiny tubes from collagen, the most abundant protein in the body, which is frequently used in bioengineering. The tubes are currently being seeded with Keller’s cholangiocytes as a prototype of lab-grown bile ducts.
Such bioengineering feats are possible thanks to microfluidics technology that enables researchers to manipulate polymers and other fluids on a tiny scale.
“Toronto has one of the highest concentrations of microfluidics researchers in the world,” says Guenther, who also directs U of T’s Centre for Microfluidic Systems in Chemistry and Biology, which designs devices for potential use in medicine. “Having that so close to teaching hospitals — there aren’t many places in the world that can offer that.”
Another engineering team, led by Molly Shoichet, a University Professor in the Department of Chemical Engineering & Applied Chemistry and the Donnelly Centre for Cellular & Biomolecular Research, is developing biomaterials capable of boosting bile duct maturation in the dish.
Implantable bile ducts remain, for now, a distant prospect, as more cell types will have to be included to faithfully mimic real-life tissue. Closer at hand is their potential to reveal what goes wrong in disease.
Working with Christine Bear, a senior scientist at SickKids and a professor in the Department of Physiology, the team will use lab-made bile ducts to shed light on cystic fibrosis, an inherited lung disease that also affects the liver.
Cystic fibrosis patients have a mutation in the protein called CFTR, which moves fluid in and out of cells, including those in the lining of bile ducts.
To Bear, being able to study CFTR in a tube rather than a flat sheet of cells, as is done in most labs, opens new possibilities for research and finding new treatments.
“The fact that we are now looking at the CFTR in a tube that could then be populated by other cells is just incredible, something that we could not have imagined before Medicine by Design was initiated,” she says.
“This can’t happen without the team effort,” says Keller whose team meets monthly to discuss progress. “We are totally dependent on each other for success.”
Making donor liver last longer
There is a pressing need to extend the shelf life of donor livers. Within hours of being removed from the body, the liver starts to disintegrate and is all too often unsuitable for transplant by the time it reaches the patient. Among the first cells to go are the so-called liver sinusoidal endothelial cells, or LSECs, the disappearance of which is thought to make liver function unravel even faster.
One way to keep the liver in mint condition longer is to replace the lost cells with lab-grown tissue. Five floors down from Keller’s lab in the Toronto MaRS Discovery Tower, a group led by Jason Fish is seeking to develop new ways to make LSECs for cell replacement therapy.
“We want to see if we can make specialized endothelial cells that we could try to engraft into the liver as it is being transplanted to try to improve organ function,” says Fish.
The problem is that endothelial cells are extremely difficult to grow. The cells’ unique, sieve-like structures for filtering blood begin to disappear as soon as the cells are put in a dish. So far, no one has succeeded in growing these cells from scratch. Fish believes the new data from McGilvray’s fresh liver samples hold the key to solving this.
“Once we understand the genes that are making these cells unique, then we want to induce those genes in cultured cells so they can become more like the specialized LSECs,” says Fish.
On the team are also: Michael Wilson, of SickKids and an associate professor in the Department of Molecular Genetics, who is analyzing gene networks in the LSECs; Michael Hoffman, a scientist at Princess Margaret Cancer Centre at UHN, and an assistant professor in the departments of Medical Biophysics and Computer Science, who is studying how the DNA is packaged inside liver cells; and Jennifer Mitchell, an associate professor in the Department of Cell and Systems Biology, who is making a tool for switching on desired genes in cultured cells.






